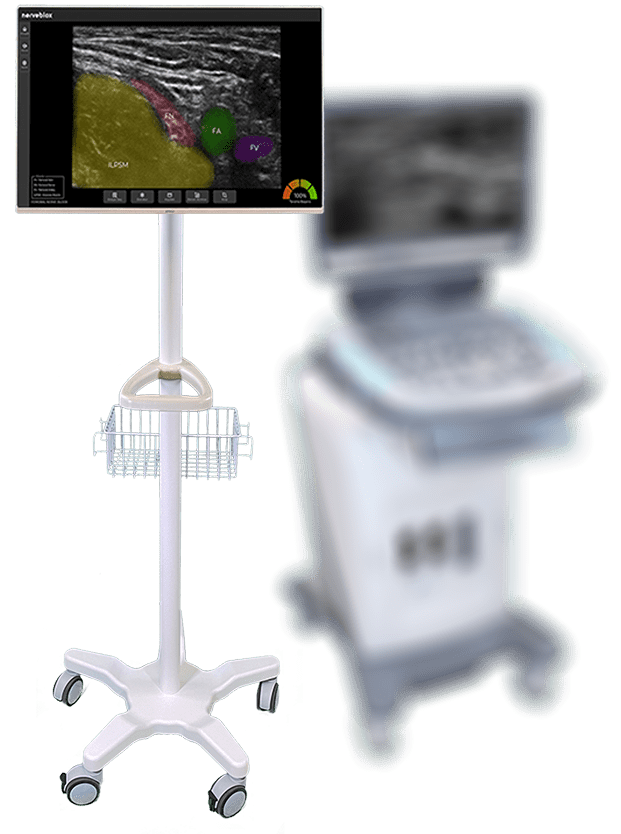
It’s perfect time to use AI in healthcare.
AI systems are able to learn incomparably faster than human beings. This allows us to develop artificial experts tutored in numerous institutes in a short time.
Nerveblox is proudly under CE approval process and simultaneously ramping up for FDA application in 2020.
What is it about?
All medical devices are subject to proof-of-value and quality before put into service. The two significant mechanisms are European Unions’s CE (Conformité Européene) and USA’s FDA (Food and Drug Administration)
In Europe, ‘European Union’ mandates that; all Member States shall take all necessary steps to ensure that devices may be placed on the market and/or put into service only if they comply with the requirements of Directives which are continuously amended on Council Directıve 93/42/EEC of 14 June 1993 This directive and amendmends are concerning medical devices, however digital forms (i.e. software) are also accepted as medical devices. The same directive defines ‘medical device’ as any instrument, apparatus, appliance, software, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of :diagnosis, monitoring, treatment, investigation, control of conception which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means.
FDA is far more sophisticated but more clear. 21st Century Cures Act (Cures Act) introduced a new paradigm “Software as a Medical Device (SaMD)” and “Software in a Medical Device (SiMD) ” classification makes the assesment more predictable for IT stakeholders in the healthcare domain. FDA intends to apply its regulatory oversight to only those software functions that are medical devices and whose functionality could pose a risk to a patient’s safety if the device were to not function as intended. FDA authorities are also clearing the fog on mobile applications and off-the-shelf (OTS) software which were used to stay uncovered areas when assurance is a concern of innovation.
Thanks to its experienced stakeholders, credible scientific approach and highly ethical data reserve, Nerveblox is committed to be continuously clear with respect to EU and USA health authorities.

AI systems are able to learn incomparably faster than human beings. This allows us to develop artificial experts tutored in numerous institutes in a short time.