It’s perfect time to use AI in healthcare.
AI systems are able to learn incomparably faster than human beings. This allows us to develop artificial experts tutored in numerous institutes in a short time.
January, 2020.
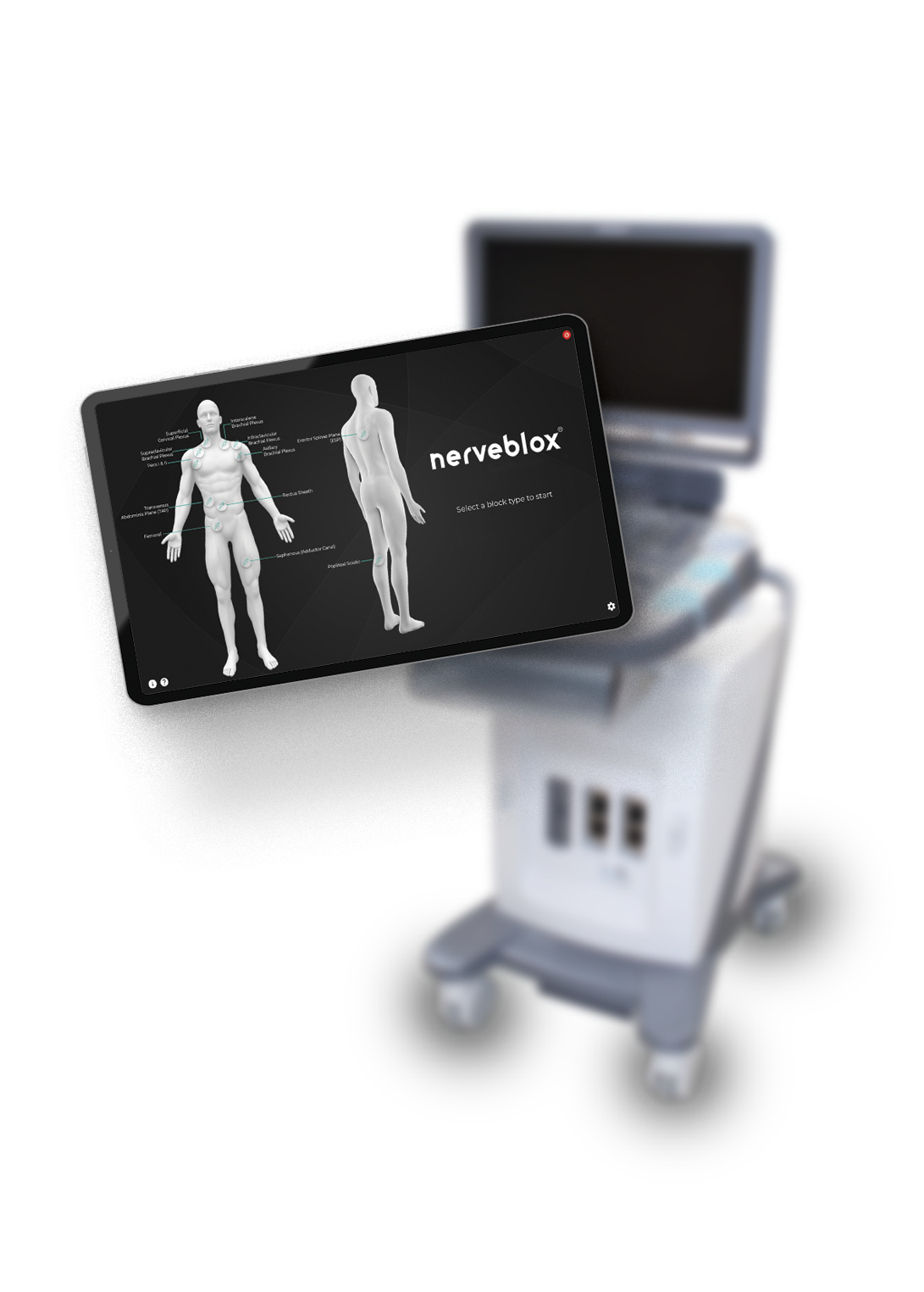
SmartAlpha (www.smartalpha.ai) is starting a clinical study for expanding the high quality dataset of Nerveblox, worlds first artificial intelligence software designed for peripheral nerve blocks. This supplementary dataset is expected to add new insights to Nerveblox’s deep learning algorithms for regional anesthesia.
With the advancements of ultrasound availability, in terms of both size and cost, ultrasound-guided nerve block has become the most efficient method of nerve blocking. This us-guided transformation introduced improved patient comfort, reduced number of complications and overall cost savings either for regional anesthesia or pain management.
Although us-guided peripheral nerve block has been accepted as state-of-the-art method for nerve blocks for the last decade, the application of this technique is still not easy for the inexperienced anesthesiologist, who are not minority worldwide.
Nerveblox is aiming to be the real-time ultrasound interpreter in the operating theatre.
In order to undertake this clinical study, SmartAlpha has obtained neccesary research ethics commiteee approvals by 2019 . The methodology to be followed is uniquely innovative and each step of the study is designed with SmartAlpha’s clinical advisory board.

AI systems are able to learn incomparably faster than human beings. This allows us to develop artificial experts tutored in numerous institutes in a short time.
Nerveblox is a CE marked medical device which indicates that it meets EU safety, health and environmental protection requirements.It is not available for sale in United States and this content does not constitute a clinical benefit nor a commercial offer.