It’s perfect time to use AI in healthcare.
AI systems are able to learn incomparably faster than human beings. This allows us to develop artificial experts tutored in numerous institutes in a short time.
26/05/2021
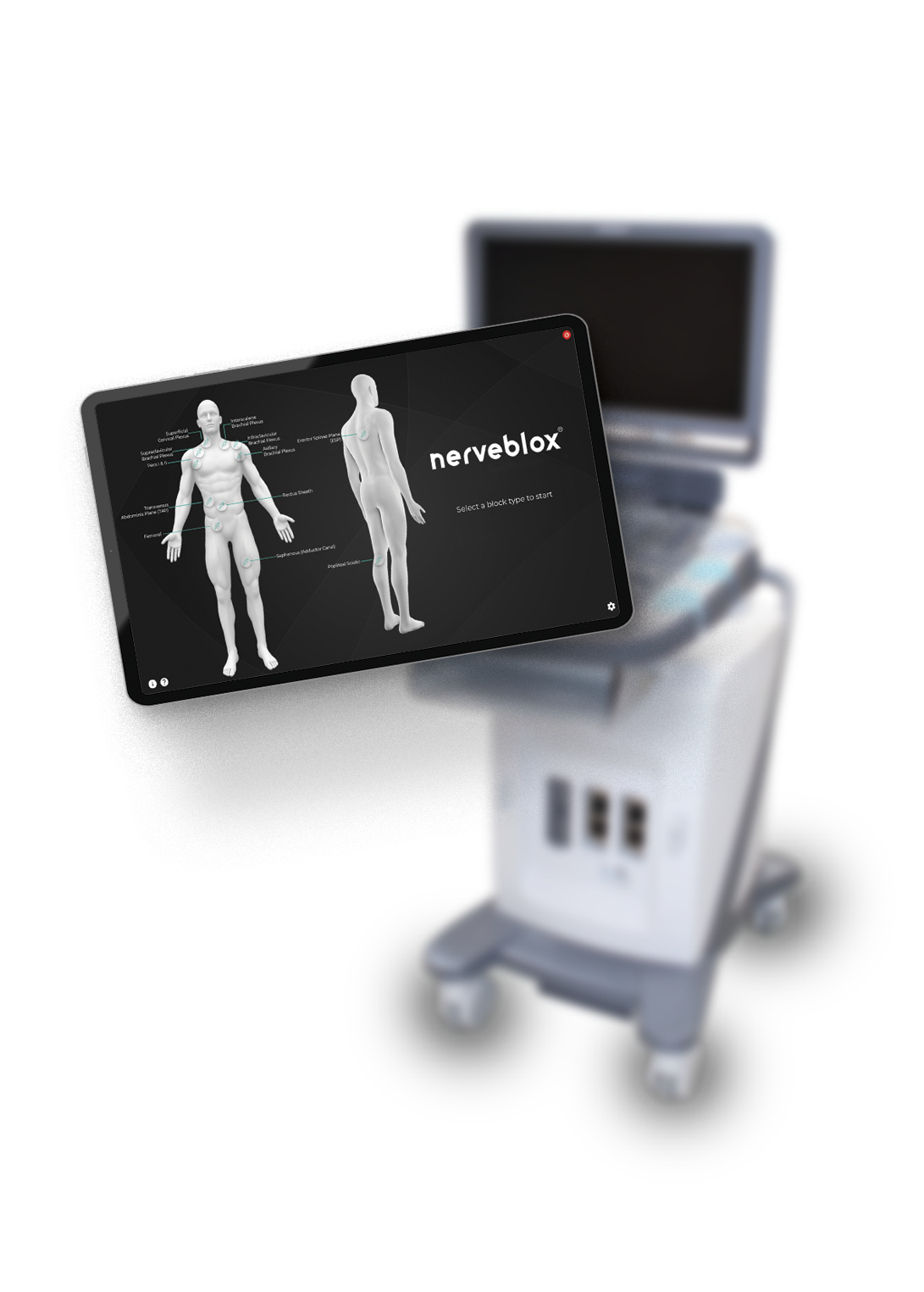
SmartAlpha announces that Nerveblox (www.nerveblox.com), the artificial intelligence driven image interpretation software designed to assist ultrasound-guided peripheral nerve blocks, has recently received CE marking approval. This certifies that Nerveblox was developed in accordance with strict medical standards and the final AI product conforms to related European medical device directives.
“Ultrasound devices are getting more and more available with their reduced cost and size, thereby ultrasound-guided interventions have seen immense growth in popularity. We clearly see increased acceptance and adoption of ultrasound usage in new use cases. This situation justifies the growing attention of physicians for our AI-driven ultrasound interpretation software. Imagine how much easier their job become if a non-radiologist physician has AI to help them more readily visualize anatomical structures during an ultrasound. “says Utku Kaya, CEO of SmartAlpha, a Turkey based AI-in-healthcare start-up founded in 2019.
Nerveblox is designed to capture real-time images from any ultrasound device. Once the physician starts the ultrasound scan, its patented algorithm gives real-time feedback on image correctness while highlighting and labelling the required anatomical structures. Nerveblox can both be used on a standalone hardware unit or deployed on suitable ultrasound devices.
With this certification, SmartAlpha was named as one of the few AI-on-ultrasound products in European market.

AI systems are able to learn incomparably faster than human beings. This allows us to develop artificial experts tutored in numerous institutes in a short time.
Nerveblox is a CE marked medical device which indicates that it meets EU safety, health and environmental protection requirements.It is not available for sale in United States and this content does not constitute a clinical benefit nor a commercial offer.